Calculate the pH of a 0.050 M solution of hydroxylamine, NH2OH. (Kb = 6.6 x 10-9) | Homework.Study.com

OneClass: For the following reaction, K>1. Classify each of the reactants and products based on th...

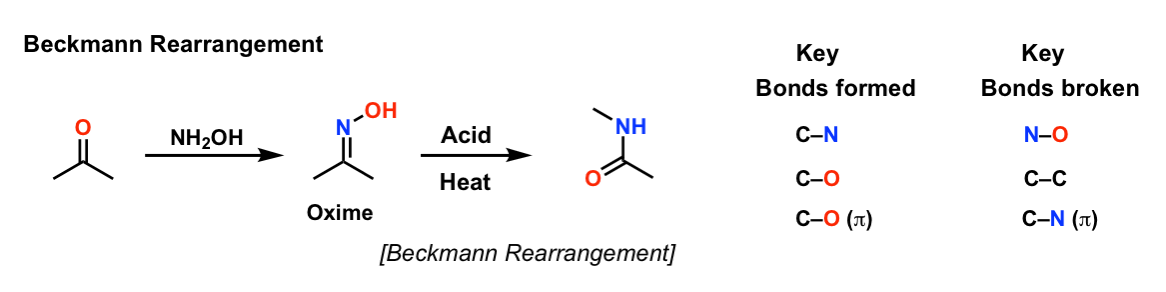
Palladium-catalyzed relay hydroaminocarbonylation of alkenes with hydroxylamine hydrochloride as an ammonia equivalent | Communications Chemistry

Photoorganocatalytic One‐Pot Synthesis of Hydroxamic Acids from Aldehydes - Papadopoulos - 2016 - Chemistry – A European Journal - Wiley Online Library

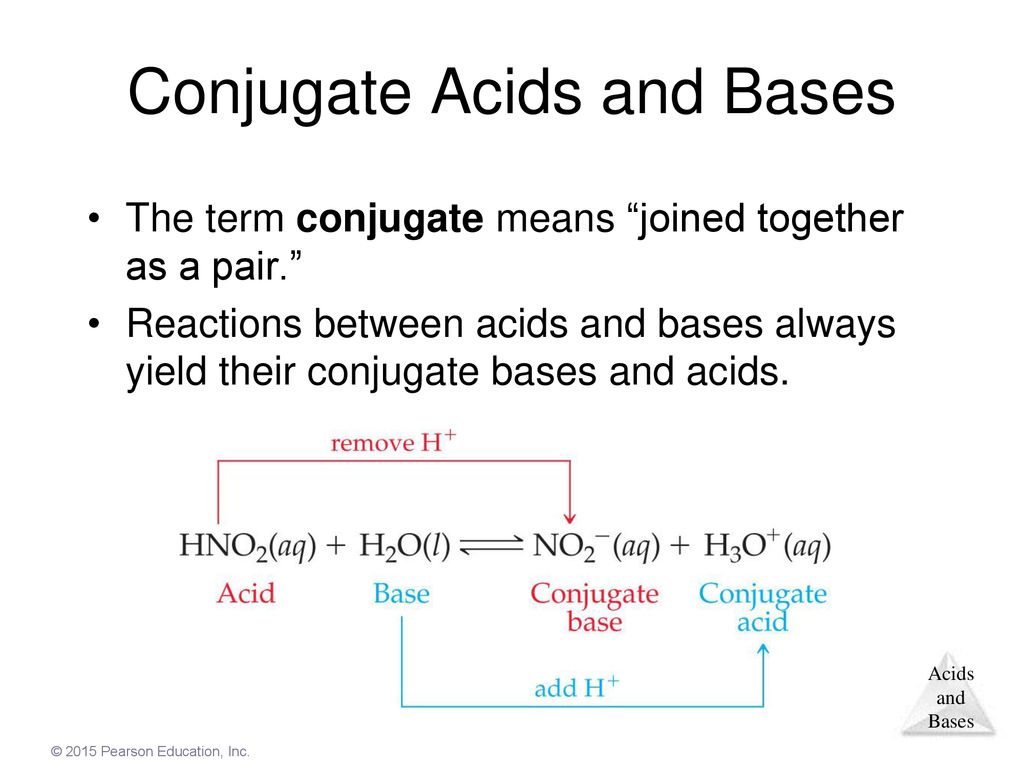
OneClass: Label the Bronsted-Lowry acid and base as well as the conjugate acid and conjugate base for...

Hydroxylamine as an Oxygen Nucleophile. Structure and Reactivity of Ammonia Oxide | Journal of the American Chemical Society

Trifluoroacetic Acid Hydroxylamine System as Organocatalyst Reagent in a One-Pot Salt Free Process for the Synthesis of Caprolactam and Amides of Industrial Interest | SpringerLink