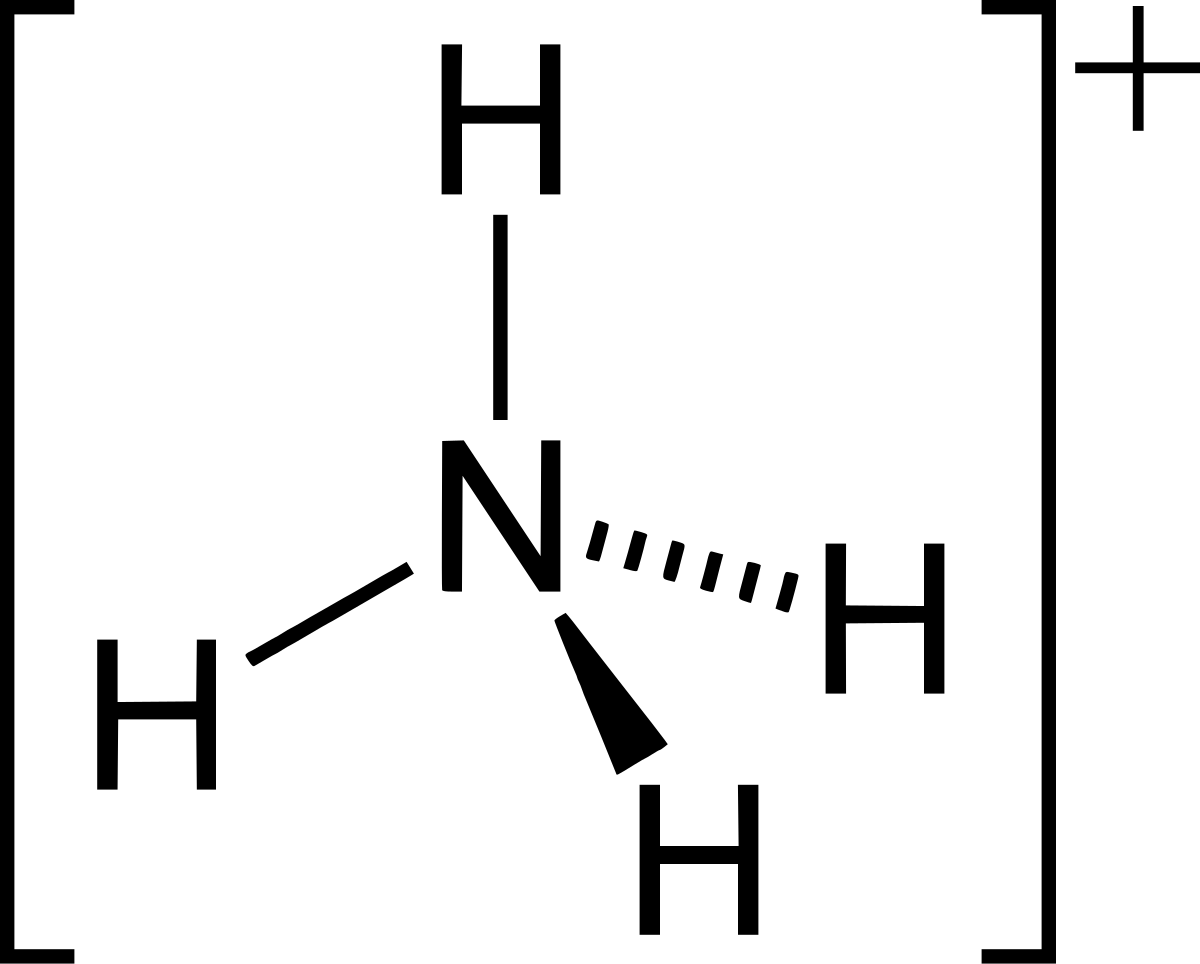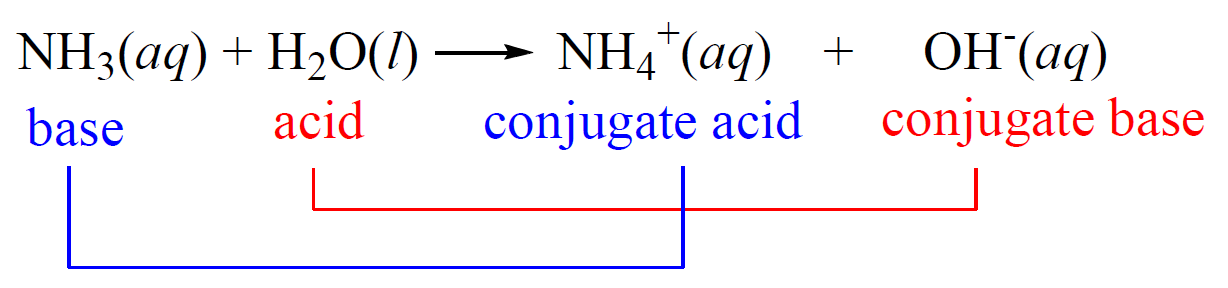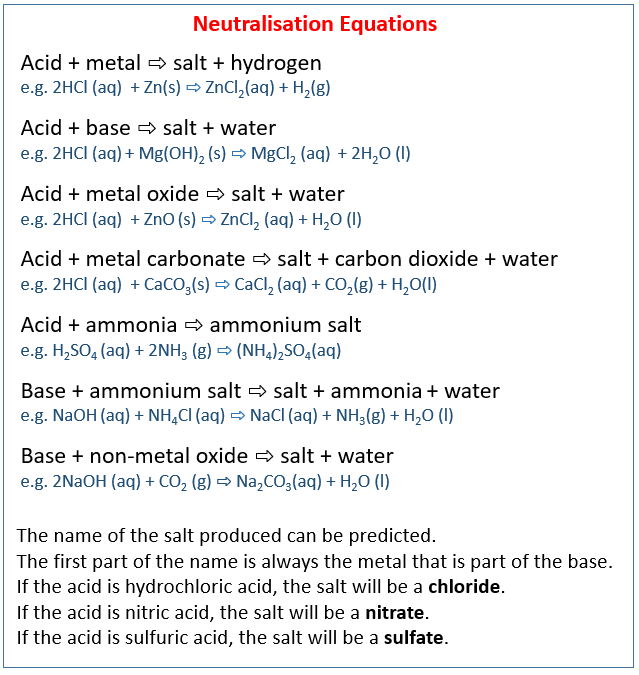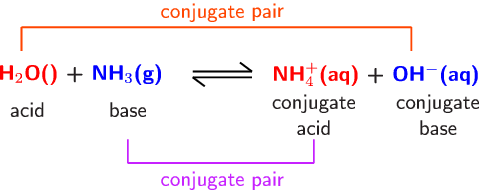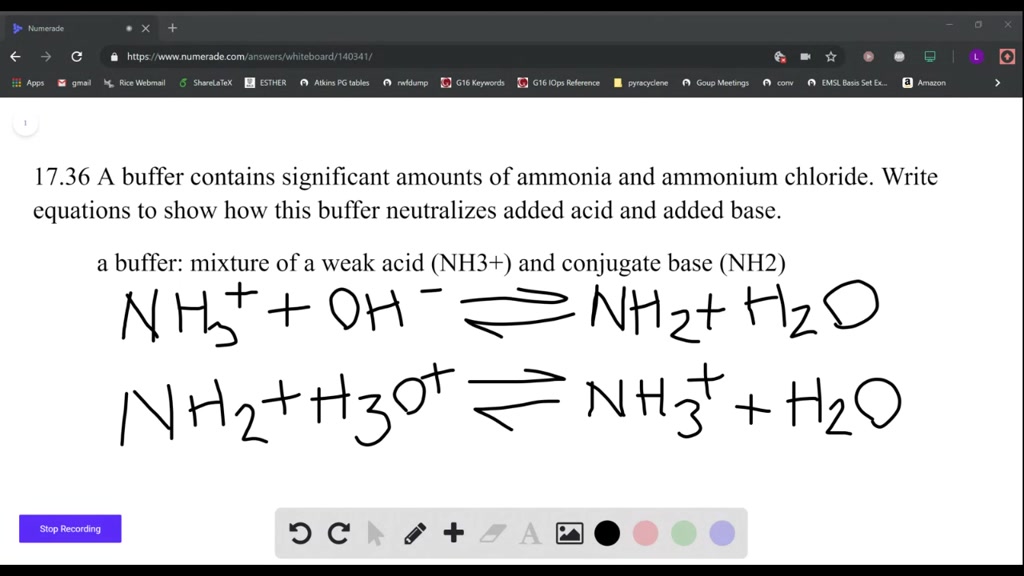
SOLVED: A buffer contains significant amounts of ammonia and ammonium ion. Write two equations, 1) showing how this buffer neutralizes added H3O+ and 2) showing how this buffer neutralizes added OH-.
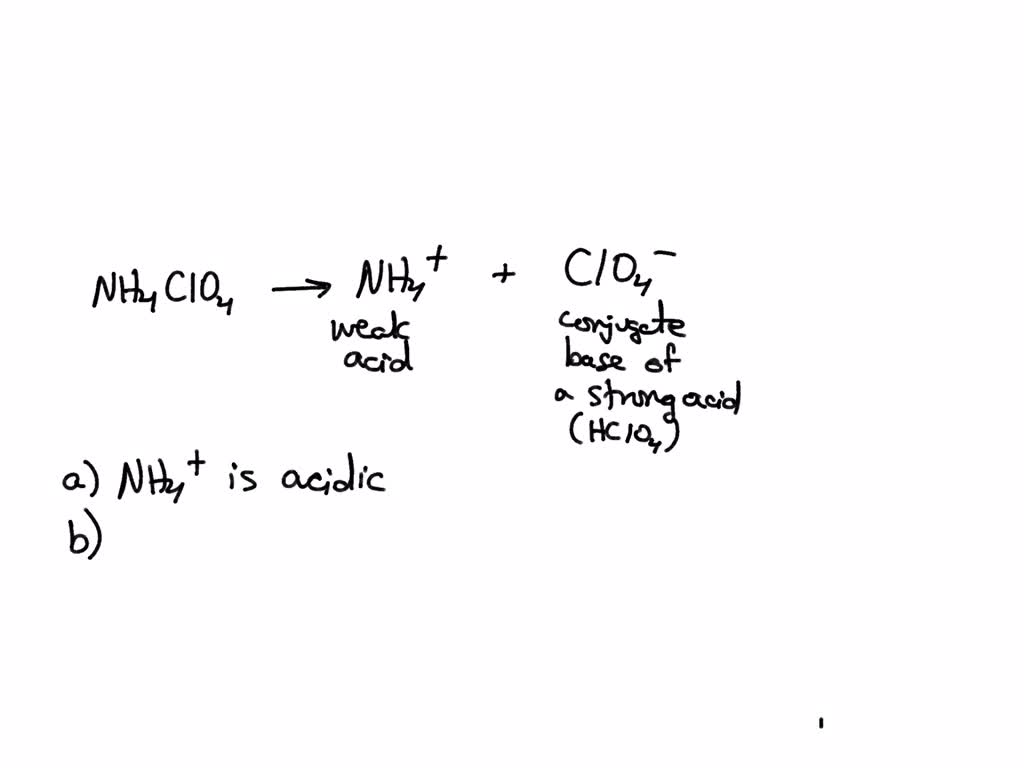
SOLVED: Consider the acid-base nature of ammonium perchlorate, NH4ClO4, when it is dissolved in water. (1) What are the acid-base properties of the cation? acidic/basic/neutral (2) What are the acid-base properties of
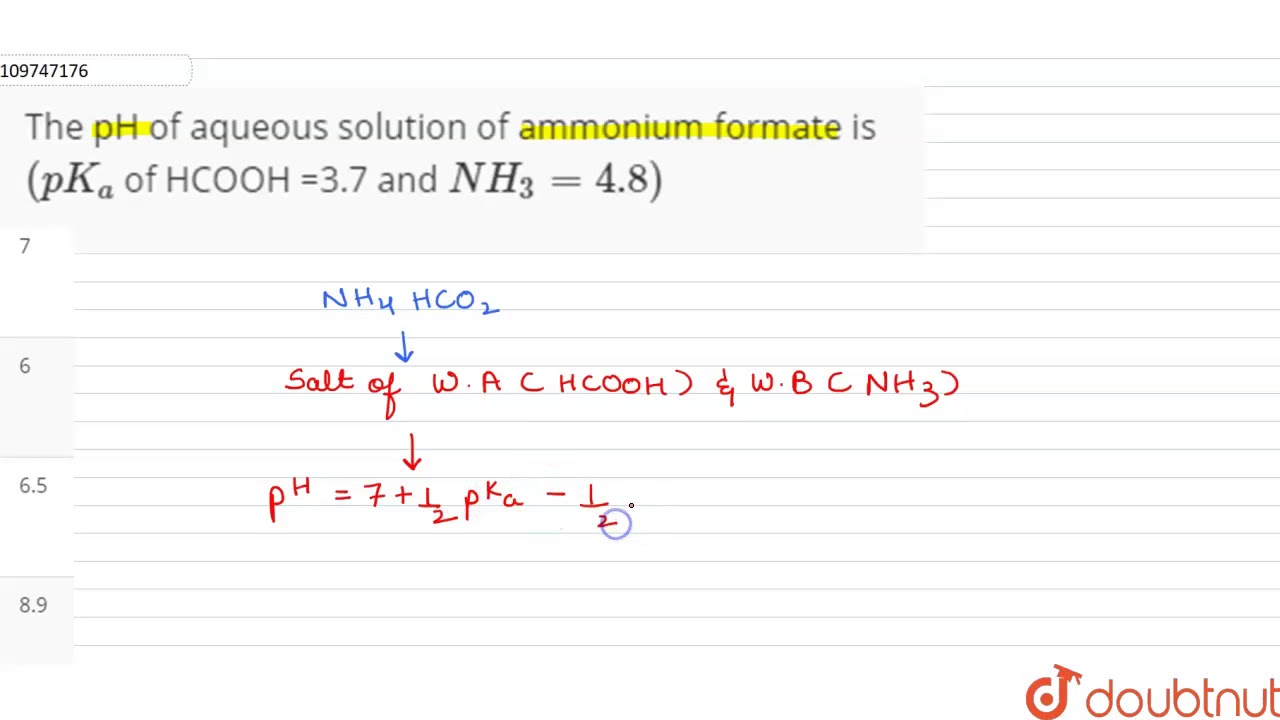
The pH of aqueous solution of ammonium formate is `(pK_(a)` of HCOOH =3.7 and `NH_(3)=4.8)` - YouTube

List of acids, bases and new ammonium-based protic ionic liquids (PILs)... | Download Scientific Diagram
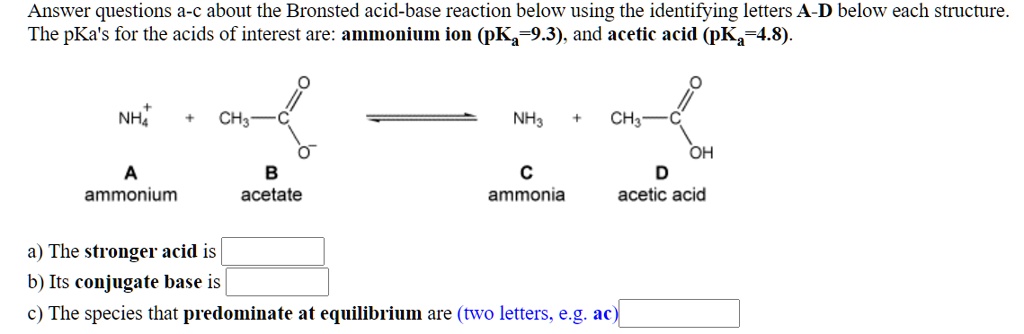
SOLVED: Answer questions a-c about the Bronsted acid-base reaction below uSing the identifying letters A-D below each structure. The pKa's for the acids of interest are: ammonium ion (pKa-9.3). and acetic acid (

Write the predominant form for Butyl Ammonium is more water-soluble or more organically soluble. | Homework.Study.com
![high school : acid and base] is that ammonium ion a weak acid or base. Because it release OH- when react with water but when I search in Internet, it shows that high school : acid and base] is that ammonium ion a weak acid or base. Because it release OH- when react with water but when I search in Internet, it shows that](https://i.redd.it/kcgf74ac4i151.jpg)
high school : acid and base] is that ammonium ion a weak acid or base. Because it release OH- when react with water but when I search in Internet, it shows that