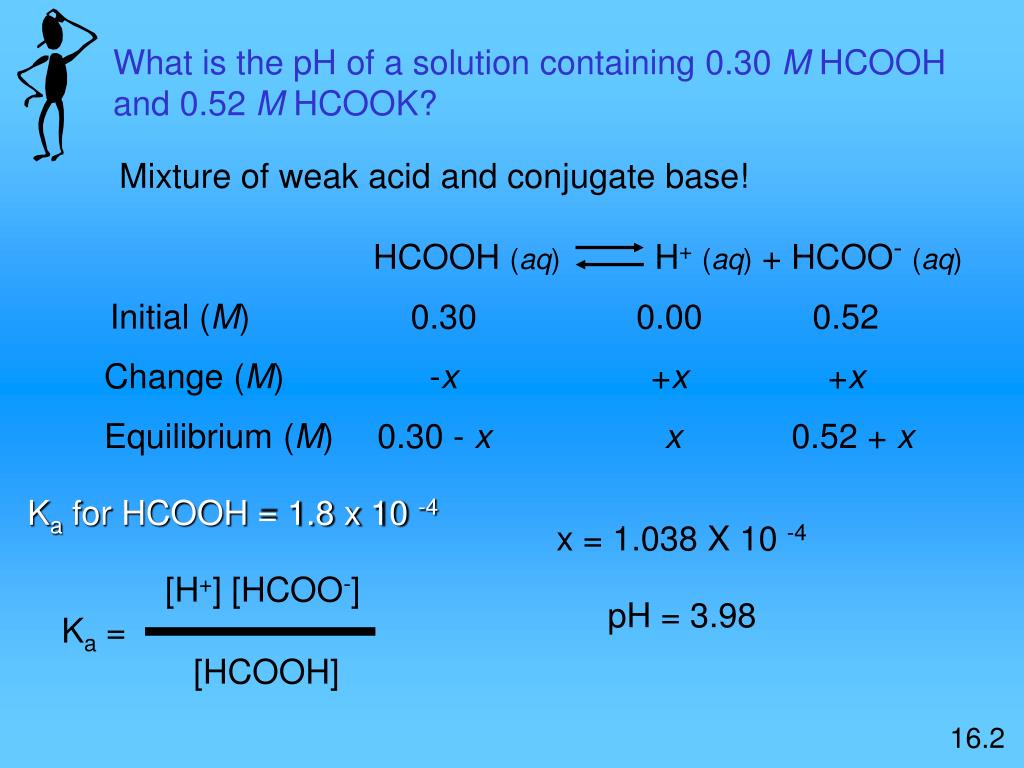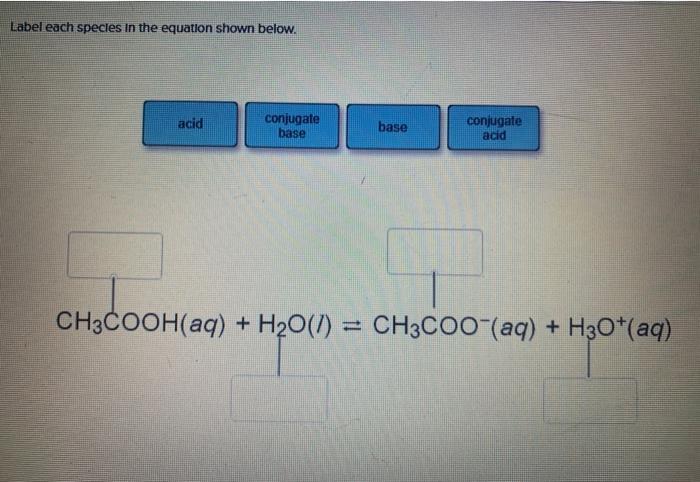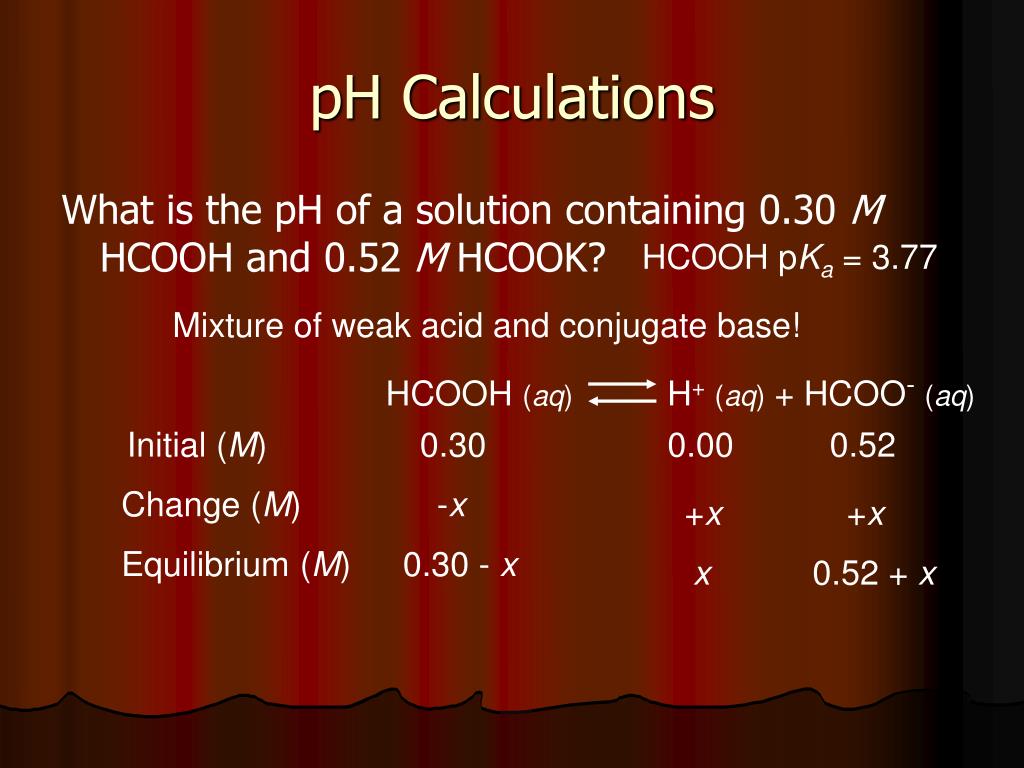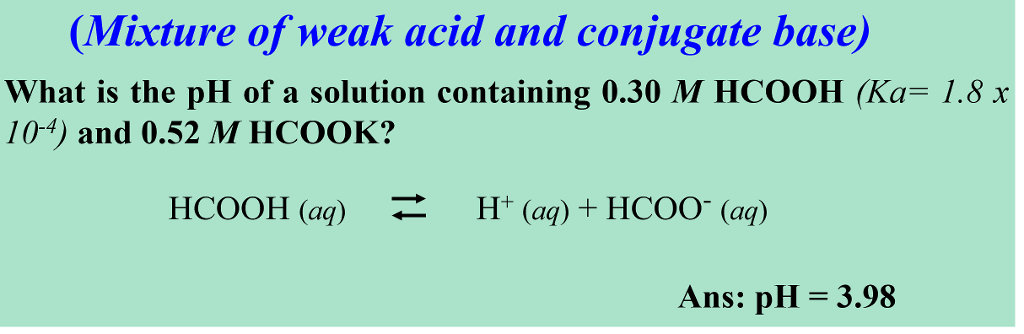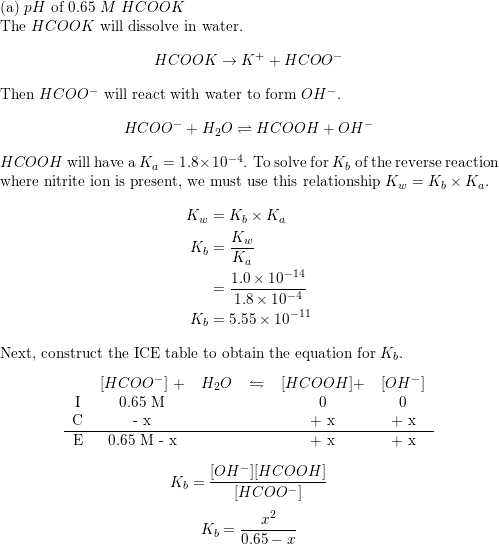
CHE 1302 Lecture Notes - Fall 2017, Lecture 12 - Equilibrium Constant, Hydrofluoric Acid, Rice Chart
SOLVED: A buffer contains HCOOH (aq) and HCOOK (aq). Which statement correctly summarizes the action of this buffer? Both HCOOH (aq) and HCOOK (aq) neutralize added acid. Both HCOOH (aq) and HCOOK (

Effect of HCOOK/Ethanol on Fe/HUSY, Ni/HUSY, and Ni–Fe/HUSY Catalysts on Lignin Depolymerization to Benzyl Alcohols and Bioaromatics | ACS Omega

Interconversion between CO2 and HCOOH under Basic Conditions Catalyzed by PdAu Nanoparticles Supported by Amine-Functionalized Reduced Graphene Oxide as a Dual Catalyst | ACS Catalysis

EHSQ (Environment,Health,Safety and Quality) : Question : Is HCOOK an acid or base or neutral ? Answer : HCOOK ( Potassium formate ) is base
A 0.05M HCOOH and 0.1M HCOOK buffer solution was diluted 100 times. How did pH change and why? - Quora
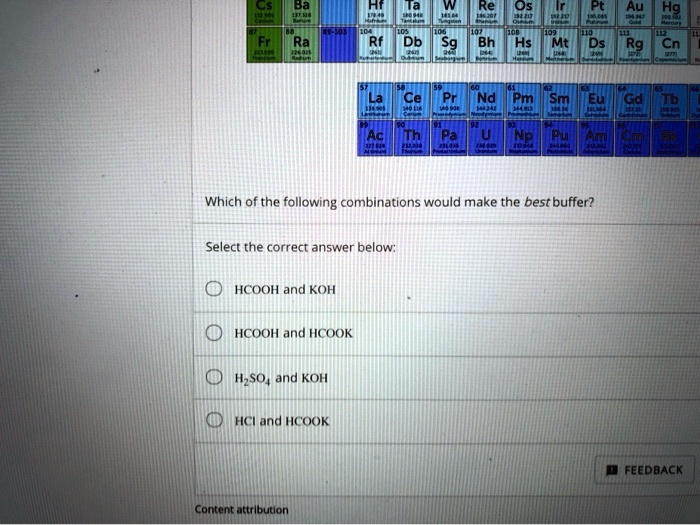
SOLVED: Os 6H Db Sg Hs Mt Ds Rg Cn Cel PN Which of the folllowing combinations would make the best buffer? Select the correct answer below HCOOH and KOH HCOOH and
In situ observation of [Mn-OOCH] by NMR. Reaction conditions: HCOOH (5... | Download Scientific Diagram

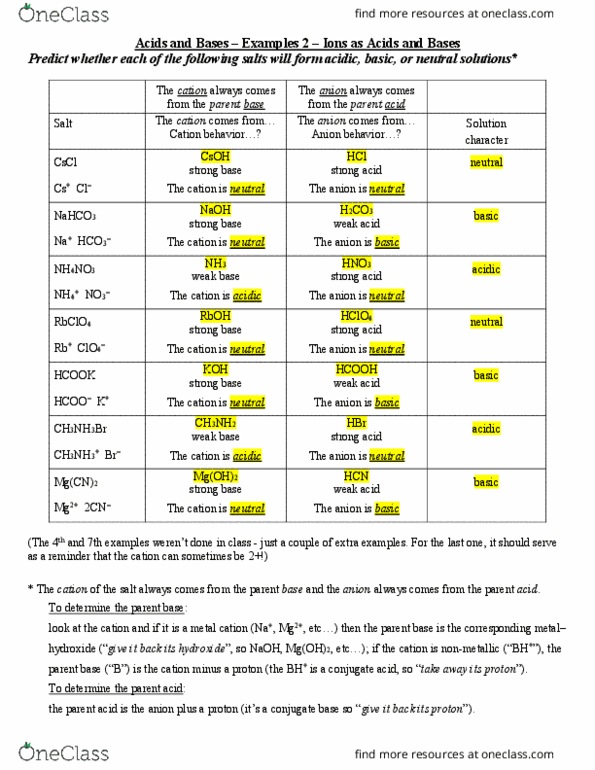
SOLVED: State whether aqueous solutions of the following salts produce acidic, basic or neutral solutions Justify your choice by referring to the specific properties of BOTH the cation and anion. You must