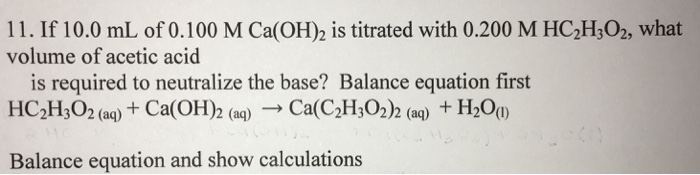
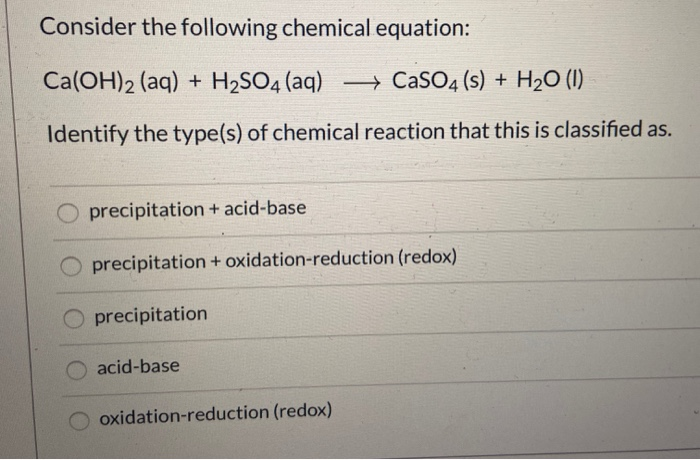
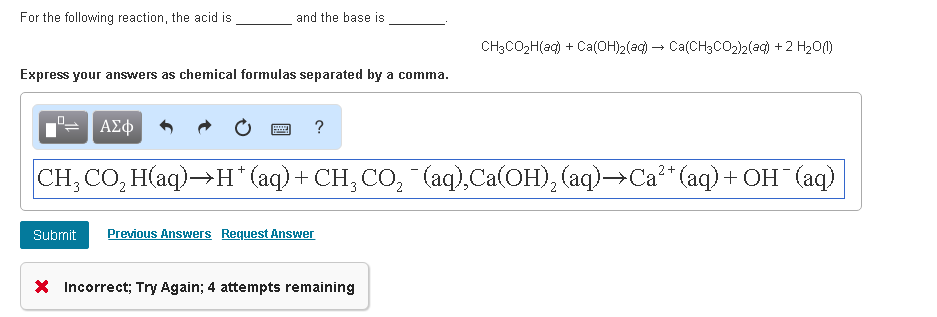
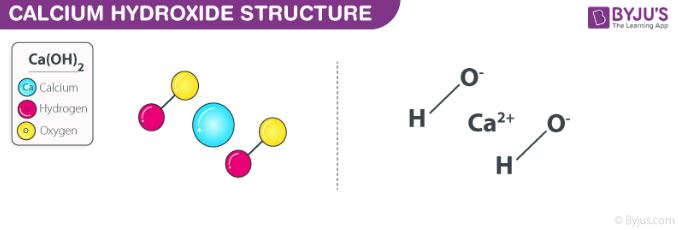
SOLVED: True or False? 1. Ca(OH)2 is a strong base and will produce 2 hydroxide ions for every calcium ion. 2. HNO2 is a diprotic acid. 3. All hydrogens are acidic, meaning
Which of the following reacts is not shown by formic acid? Reaction with Ca( OH)2 Reaction with I2 / Red P Reaction with NaHCO3 Reaction with C2H5OH

PPT - STRONG BASES LiOH, NaOH, KOH, RbOH, CsOH, Ca(OH) 2 , Sr(OH) 2 , Ba(OH) 2 PowerPoint Presentation - ID:5872807
![SOLVED: What is a correct charge balance equation for a 0.1 M solution of Ca (OH); strong base? [Cat+] [HT] [OH:] 2 [Cat+] [H] = [OH] + [CaOH:] [Cat+] [H ] = [OH:] 2 [Ca" [Ht] = [OH:] SOLVED: What is a correct charge balance equation for a 0.1 M solution of Ca (OH); strong base? [Cat+] [HT] [OH:] 2 [Cat+] [H] = [OH] + [CaOH:] [Cat+] [H ] = [OH:] 2 [Ca" [Ht] = [OH:]](https://cdn.numerade.com/ask_images/0aaebe4b86504103aa4b2a54d39b5253.jpg)
SOLVED: What is a correct charge balance equation for a 0.1 M solution of Ca (OH); strong base? [Cat+] [HT] [OH:] 2 [Cat+] [H] = [OH] + [CaOH:] [Cat+] [H ] = [OH:] 2 [Ca" [Ht] = [OH:]
![Calcium hydroxide is a strong base. Compute `[Ca^(2+)]` and `[OH^(-)]` for a solution that is pr... - YouTube Calcium hydroxide is a strong base. Compute `[Ca^(2+)]` and `[OH^(-)]` for a solution that is pr... - YouTube](https://i.ytimg.com/vi/wtPIrhTYyLE/maxresdefault.jpg)