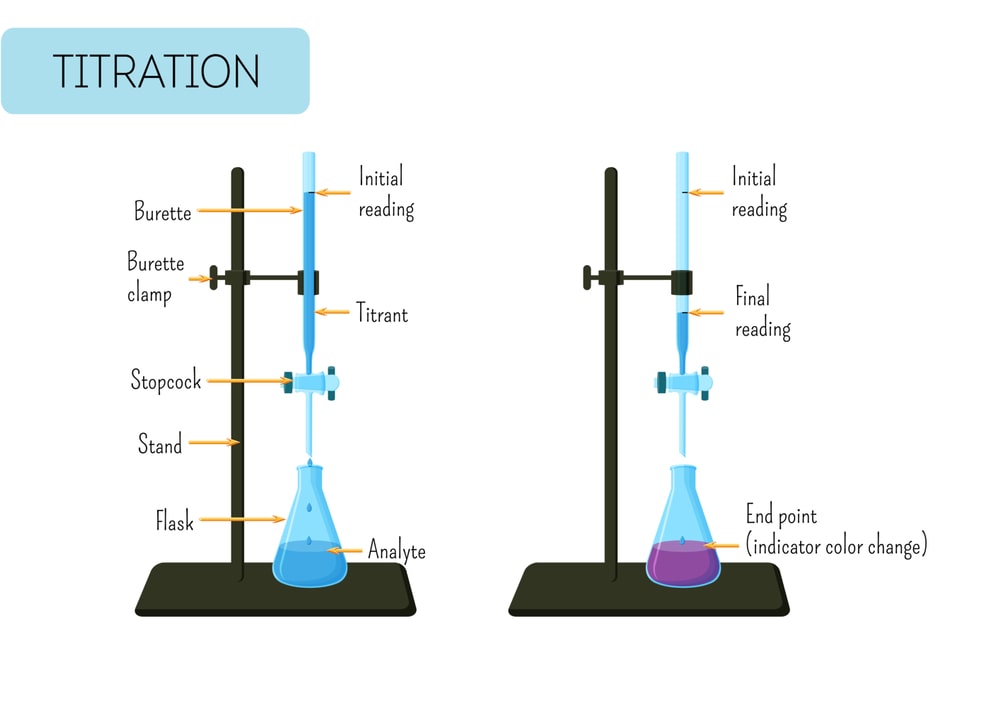
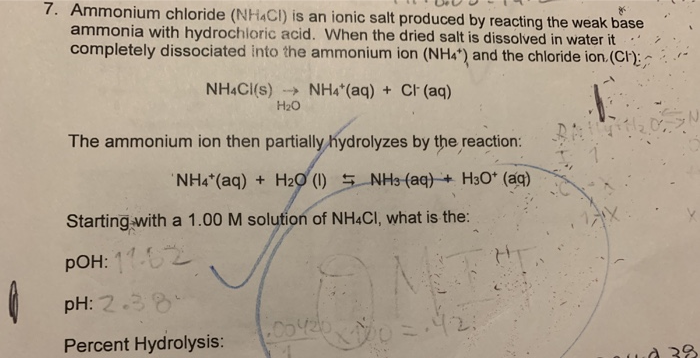CHEM 1332 (A.M. Guloy) CHEMICAL EQUILIBRIA--ACID/BASE Acid/base problems may fall into 4 categories: strong acid/base, weak acid

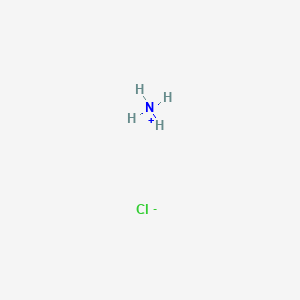
Identity the acid and base for ammonium chloride salt. state the nature of this salt and mention its - Brainly.in

SOLVED:A buffer contains significant amounts of ammonia and ammonium chloride. Write equations showing how this buffer neutralizes added acid and added base.

Question Video: Selecting the Correction Equation for the Reversible Reaction of Hydrogen Chloride and Ammonia to Make Ammonium Chloride | Nagwa
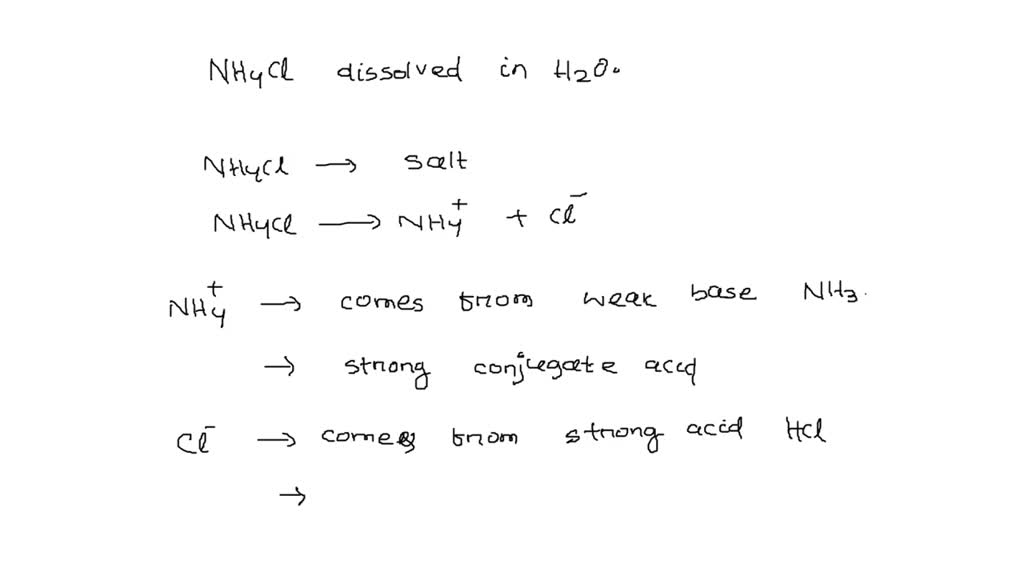
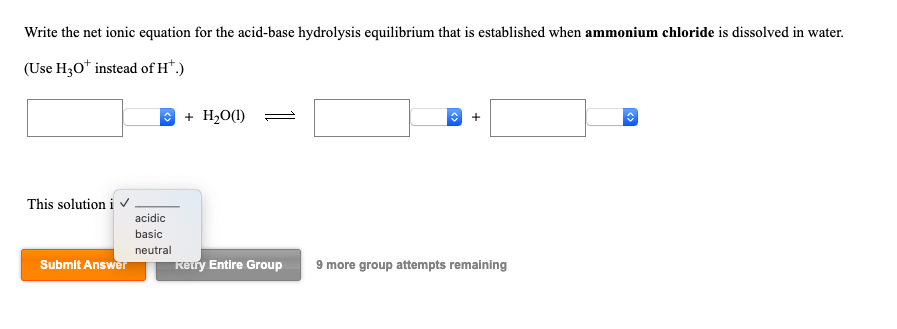
SOLVED: Write the net ionic equation for the acid-base hydrolysis equilibrium that is established when ammonium chloride is dissolved in water. (Use H3O+ instead of H+.) + H2o(l) = + is the

Ammonium-chloride-assay - Assay Of Ammonium Chloride AIM: To determine the percentage purity of - Studocu


![Solved BUFFERS 10. An ammonium[NH3]/ammonium chloride | Chegg.com Solved BUFFERS 10. An ammonium[NH3]/ammonium chloride | Chegg.com](https://media.cheggcdn.com/media/98f/s907x655/98fc93a3-c6f6-4a89-8acb-45d7d9f5eb4d/image.png)